| Product Name | Necrostatin |
| Description |
Necroptosis inhibitor |
| Purity | >98% |
| CAS No. | 4311-88-0 |
| Molecular Formula | C13H13N3OS |
| Molecular Weight | 259.33 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | Soluble to 15 mM in ethanol, and to 30 mM in DMSO |
| Source | Synthetic |
| Appearance | Light Yellow Solid |
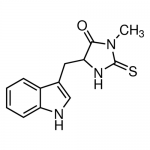
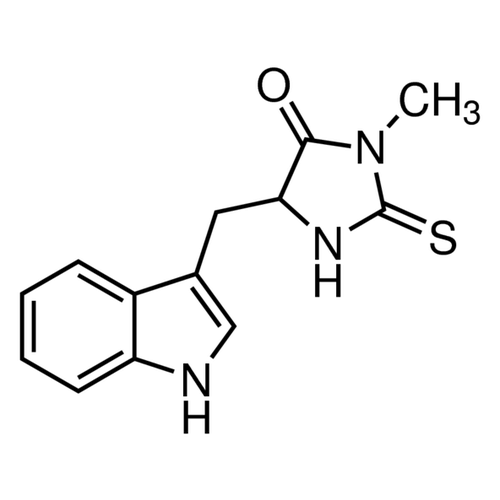
| SMILES | CN1C(=O)C(NC1=S)CC2=CNC3=CC=CC=C32 |
| InChI | InChI=1S/C13H13N3OS/c1-16-12(17)11(15-13(16)18)6-8-7-14-10-5-3-2-4-9(8)10/h2-5,7,11,14H,6H2,1H3,(H,15,18) |
| InChIKey | TXUWMXQFNYDOEZ-UHFFFAOYSA-N |
| Safety Phrases |
Classification: Caution: Substance not yet fully tested. Safety Phrases: S22 - Do not breathe dust S24/25 - Avoid contact with skin and eyes S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection |
| Cite This Product | Necrostatin (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-213) |
Biological Description
| Alternative Names | 5-(1H-Indol-3-ylmethyl)-3-methyl-2-thioxo-4-imidazolidinone, Necrostatin-1 |
| Research Areas | Apoptosis, Cancer |
| PubChem ID | 2828334 |
| Scientific Background | Necrostatin-1 is a specific inhibitor of necroptosis that reduces ischemic tissue damage in stroke models (1). It has also been recently confirmed to be an allosteric RIP1 kinase inhibitor, preotective in NMDa-mediated excitotoxicity and acute pathologies (2,3). |
| References |
1. You Z., et al. (2008) J Cereb Blood Flow Metab. 28(9): 1564-1573. 2. Vandenabeele P., et al. (2010) Nat Rev Mol Cell Biol. 11:700-714. 3. Degterev A., et al. (2008) Nat Chem Biol. 4: 313-321. |

StressMarq Biosciences :
Based on validation through cited publications.