| Product Name | Tafamidis |
| Description |
Transthyretin Dissociation Inhibitor |
| Purity | >98% (TLC); NMR (Conforms) |
| CAS No. | 594839-88-0 |
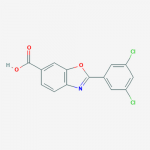
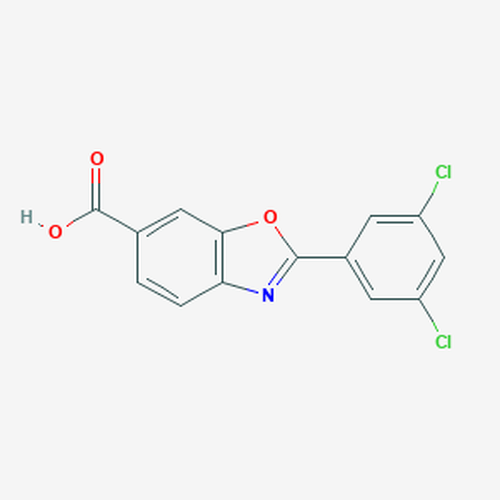
| Molecular Formula | C14H7Cl2NO3 |
| Molecular Weight | 308.1 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | May be dissolved in DMSO (25 mg/ml, warm) |
| Source | Synthetic |
| Appearance | Off-white powder |
| SMILES | C1=CC2=C(C=C1C(=O)O)OC(=N2)C3=CC(=CC(=C3)Cl)Cl |
| InChI | InChI=1S/C14H7Cl2NO3/c15-9-3-8(4-10(16)6-9)13-17-11-2-1-7(14(18)19)5-12(11)20-13/h1-6H,(H,18,19) |
| InChIKey | TXEIIPDJKFWEEC-UHFFFAOYSA-N |
| Safety Phrases |
Classification: Danger- Irritant and Health Hazards GHS Hazard Statements: H315: Causes skin irritation H319: Causes serious eye irritation H335: May cause respiratory irritation H360: May damage fertility or the unborn child Precautionary Statements: P201, P202, P261, P264, P271, P280, P281, P302+P352, P304+P340, P305+P351+P338, P308+P313, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, and P501 |
| Cite This Product | Tafamidis (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-584) |
Biological Description
| Alternative Names | 2-(3,5-Dichlorophenyl)-1,3-Benzoxazole-6-Carboxylic Acid, FX-1006, 2-(3,5-Dichlorophenyl)-6-benzoxazole carboxylic acid |
| Research Areas | Alzheimer's Disease, Axon Markers, Cell Markers, Cell Signaling, Cytoskeleton, Microtubules, MT Associated Proteins, Neurodegeneration, Neuron Markers, Neuroscience, Tangles & Tau |
| PubChem ID | 11001318 |
| Scientific Background | Tafamidis functions as a chaperone that stabilizes the correctly folded tetrameric form of the transthyretin (TTR) protein by binding in one of the two thyroxine-binding sites of the tetramer (1). In drug form, it is used to delay impairment of peripheral nerve function in adults with familial amyloid polyneuropathy. |
| References | 1. Said G, Grippon S, Kirkpatrick P. (2012). Nature Reviews. Drug Discovery. 11(3): 185–186. |

Reviews
There are no reviews yet.