| Product Name | Thalidomide |
| Description |
E3 UB ligase inhibitor |
| Purity | >98% |
| CAS No. | 50-35-1 |
| Molecular Formula | C13H10N2O4 |
| Molecular Weight | 258.2 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | Soluble in 5 mg/ml DMSO |
| Source | Synthetic |
| Appearance | White Solid |
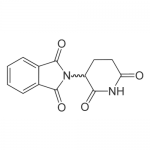
| SMILES | C1CC(=O)NC(=O)C1N2C(=O)C3=CC=CC=C3C2=O |
| InChI | InChI=1S/C13H10N2O4/c16-10-6-5-9(11(17)14-10)15-12(18)7-3-1-2-4-8(7)13(15)19/h1-4,9H,5-6H2,(H,14,16,17) |
| InChIKey | UEJJHQNACJXSKW-UHFFFAOYSA-N |
| Safety Phrases |
Classification: Very toxic material causing immediate and serious toxic effects. Highly toxic teratogen. Safety Phrases: S22 - Do not breathe dust S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection S24/25- Avoid contact with skin and eyes Hazard Statements: H302 – Harmful if swallowed H360 – May damage fertility or the unborn child Precautionary Statements: P201 – Obtain special instructions before use. P308 + P313 – If exposed or concerned: Get medical advice/ attention. |
| Cite This Product | Thalidomide (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-336) |
Biological Description
| Alternative Names | (±)-2-(2,6-Dioxo-3-piperidinyl)-1H-isoindole-1,3(2H)-dione, N-(2,6-dioxo-3-piperidinyl)phthalimide |
| Research Areas | Apoptosis, Cancer |
| PubChem ID | 5426 |
| Scientific Background | Binds to the E3 ubiquitin ligase complex formed between CRBN, DDB1 and Cul4A inhibiting ligase activity. Inhibits TNF synthesis and FGF-induced angiogenesis. Cell permeable. Caution: Teratogenic. |
| References |
1. Ito T., et al. (2010) Science. 327:1345. 2. Weglicki W.B., et al. (1993) Mol. Cell. Biochem. 129:195. 3. D’Amato R.J., et al. (1994) Proc. Natl. Acad. Sci. USA. 91:4082. |

Reviews
There are no reviews yet.