| Product Name | Trichostatin A |
| Description |
HDAC inhibitor |
| Purity | >98% |
| CAS No. | 58880-19-6 |
| Molecular Formula | C17H22N2O3 |
| Molecular Weight | 302.37 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | Soluble to 10 mM in ethanol and to 50 mM in DMSO |
| Source | Synthetic |
| Appearance | Beige Solid |
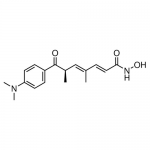
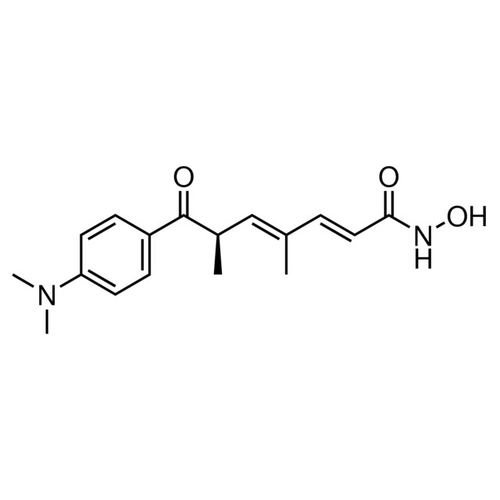
| SMILES | C[C@H](/C=C(C)/C=C/C(=O)NO)C(=O)C1=CC=C(C=C1)N(C)C |
| InChI | InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+ |
| InChIKey | RTKIYFITIVXBLE-WKWSCTOISA-N |
| Safety Phrases |
Classification: Caution: Substance not yet fully tested. Safety Phrases: S22 - Do not breathe dust S24/25 - Avoid contact with skin and eyes S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection Risk Phrases: R20/21/22 - Harmful by inhilation, in contact with skin and if swallowed R68 - Possible risk of irreversible effects Hazard Phrases: H302-H312-H315-H317-H319-H332-H335 Precautionary Phrases: P261-P280-P305 + P351 + P338 |
| Cite This Product | Trichostatin A (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-254) |
Biological Description
| Alternative Names | (2E,4E,6R)-7-[4-(Dimethylamino)phenyl]-N-hydroxy-4,6-dimethyl-7-oxo-2,4-heptadienamide |
| Research Areas | Apoptosis, Cancer, Cancer Growth Inhibitors, HDAC Inhibitors |
| PubChem ID | 444732 |
| Scientific Background | Trichostatin A is an antifungal and selectively inhibits the class I and II mammalian histon deacetlyase families of enzymes (1). TSA inhibits the eukayotic cell cycle during the beginning of the growth stage. It can be used to alter gene expression but interfering with the removal of acetyl groups thereby altering the ability of DNA transcription factors to access the DNA inside chromatin (2). It also has anti-cancer properties (3). |
| References |
1. Vanhaecke T., Papeleu P., Elaut G., Rogiers V. (2004) Curr Med Chem. 11(12): 1629-1643. 2. Yoshida, et al. (1990) J Biol Chem. 165: 17174. 3. Drummond D.C., et al. (2005) Annu Rev Pharmacol Toxicol. 45: 495-528. |

Reviews
There are no reviews yet.