| Product Name | Verrucarin A |
| Description |
Modulates Amyloid-beta production in Alzheimer’s neurons |
| Purity | >98% (HPLC); NMR (conforms) |
| CAS No. | 3148-09-2 |
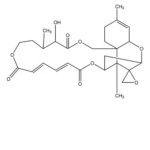
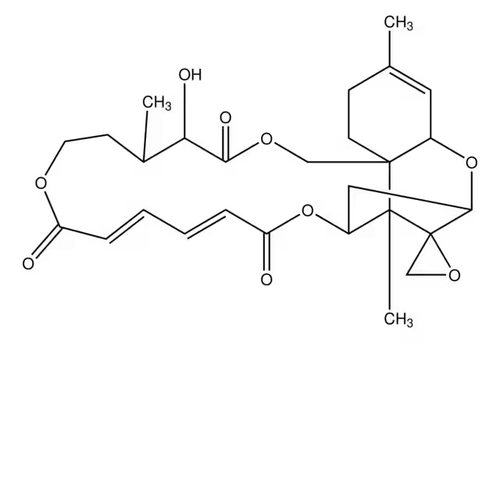
| Molecular Formula | C27H34O9 |
| Molecular Weight | 502.6 |
| Field of Use | Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only. |
Properties
| Storage Temperature | -20ºC |
| Shipping Temperature | Shipped Ambient |
| Product Type | Inhibitor |
| Solubility | May be dissolved in DMSO (25 mg/ml) |
| Source | Synthetic |
| Appearance | Off-white powder |
| SMILES | CC1CCOC(=O)C=CC=CC(=O)OC2CC3OC4C=C(C)CCC4(COC(=O)C1O)C2(C)C35CO5 |
| InChI | 1S/C27H34O9/c1-16-8-10-26-14-33-24(31)23(30)17(2)9-11-32-21(28)6-4-5-7-22(29)36-18-13-20(35-19(26)12-16)27(15-34-27)25(18,26)3/h4-7,12,17-20,23,30H,8-11,13-15H2,1-3H3/b6-4+,7-5+ |
| InChIKey | NLUGUZJQJYVUHS-YDFGWWAZSA-N |
| Safety Phrases |
Classification: Danger. Hazard Statements H300 (100%): Fatal if swallowed [Danger Acute toxicity, oral] H310 (100%): Fatal in contact with skin [Danger Acute toxicity, dermal] H330 (100%): Fatal if inhaled [Danger Acute toxicity, inhalation] Precautionary Statement Codes:, P260 - P262 - P264 - P280 - P302 + P352 + P310 - P304 + P340 + P310 |
| Cite This Product | Verrucarin A (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SIH-614) |
Biological Description
| Alternative Names | Muconomycin-A; 379-Y; Glutinosin-I; Myrothecin-I; NSC-126728 |
| Research Areas | Alzheimer's Disease, Cancer, Cell Signaling, Growth Factors, Neurodegeneration, Neuroscience, TNF |
| PubChem ID | 6326658 |
| Scientific Background | Verrucarin A is a macrocyclic trichothecene mycotoxin from Myrothecium sp. It is being investigated as it modulates amyloid-beta production in Alzheimer's patients neurons (1). It is a potent and selective inhibitor of the steroid receptor coactivator-3 and selectively down-regulates its expression (2). It is cytotoxic to multiple types of cancer cells at low nM concentrations (IC50=4.9 nM for HepG2 cells) but not toward normal liver cells (2,3). It enhances TNFα-induced (4) and TRAIL-induced apoptosis (5). It reduces phosphorylation of IREα and inhibits tunicamycin-induced ER stress in FaO rat liver cells (6). |
| References |
1. Kondo T., et al. (2022) Sci Rep. 12(1): 2690: https://pubmed.ncbi.nlm.nih.gov/35236875/ 2. F Yan et al. PLOS One 2014 9(4):e95243 3. K Palanivel et al. Tumour Biol. 2014 35:10159 4. RG Jayasooriya et al. Environ. Toxicol. Pharmacol. 2013 36:303 5. D-O Moon et al. Toxicol. In Vitro 2013 27:257 6. EY Bae et al. Molecules 2015 20:8988 |

Reviews
There are no reviews yet.