Cell Culture Assay
The following protocol was used to generate the ICC images of neurons treated with type 1 and type 2 alpha-synuclein preformed fibrils (PFFs). Primary rat hippocampal neurons show lewy body inclusion formation when treated with type 1 alpha-synuclein PFFs but not when treated with type 2 alpha-synuclein PFFs.
- Hippocampal neurons harvested from postnatal day 1 Sprague Dawley rat pups and plated at 80,000 cells per well on a poly-D-lysine and laminin-coated plate.
- Preformed fibrils were sonicated for 1 hour in a water-bath sonicator immediately prior to treatment.
- Neurons were treated with preformed fibrils at 4 µg/mL. Control wells with vehicles were maintained.
- All wells underwent a 50% media change after 7 days.
- All wells were fixed with 4% formaldehyde for 20 minutes 7 days after the media change (day-in-vitro 14 after treatment).
- The formaldehyde was washed off with three washes of filtered 10 mM phosphate-buffered saline (10 minutes each).
- Cells were then blocked with 1:1 PBS: LiCOR Odyssey Block (LiCOR, 927-40010) and 30 uL/mL of 0.1% triton-X 100 for 30 mins.
- After 30 mins, primary antibodies diluted in 1:1 PBS: LiCOR Odyssey Block (with 30 uL/mL of 0.1% triton-X 100) were added to the wells and incubated overnight at 4 degrees.
- The next day, the primary antibody solution was washed off with three washes of filtered 10 mM phosphate-buffered saline (10 minutes each).
- The secondary antibody was diluted in 1:1 PBS: LiCOR Odyssey Block (with 30 uL/mL of 0.1% triton-X 100) and added to the wells for 60 mins in the dark.
- After 60 mins, the secondary antibody solution was washed off with three washes of filtered 10 mM phosphate-buffered saline (10 minutes each).
- The plate was then imaged on an epifluorescent microscope (Olympus IX73, B&B Microscopes) at 20X objective.
- All images were captured at the same fixed scaling and same exposure times.
Primary antibody: Anti-pSer129
Counterstain: Hoechst reagent
Counterstain dilution: 1:3000
Counterstain incubation time: 1 hr
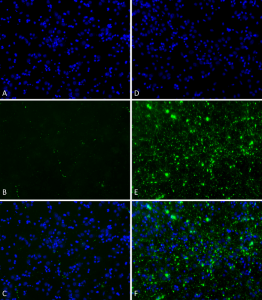
Primary rat hippocampal neurons show lewy body inclusion formation when treated with Type 1 Alpha Synuclein Protein Preformed Fibrils (SPR-322) at 4 µg/ml (D-F), but not when treated with Type 2 Alpha Synuclein Protein Preformed Fibrils (SPR-317) at 4 µg/ml (A-C). Tissue: Primary hippocampal neurons. Species: Sprague-Dawley rat. Fixation: 4% formaldehyde from PFA. Primary Antibody: Mouse anti-pSer129 Antibody at 1:1000 24 hours at 4°C. Secondary Antibody: FITC Goat Anti-Mouse (green) at 1:700 for 1 hours at RT. Counterstain: Hoechst (blue) nuclear stain at 1:4000 for 1 hour at RT. Localization: Lewy body inclusions. Magnification: 20x.
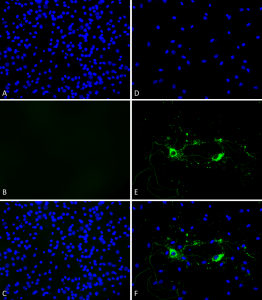
Primary rat hippocampal neurons (DIV16) show lewy body inclusion formation and loss of cells when treated with Type 1 mouse Alpha Synuclein Protein Preformed Fibrils (SPR-324) at 4 µg/ml (D-F) on DVI2, but not when treated with a control (A-C). Tissue: Primary hippocampal neurons. Species: Sprague-Dawley rat. Fixation: 3% formaldehyde from PFA for 20 min. Blocker: 1:1 PBS:LiCOR Odyssey Block (LiCOR, 927-40010) and 30 uL/mL of 0.1% triton-X 100 for 30 min. Primary Antibody: Mouse anti-pSer129 Antibody (1:1000) and Rabbit anti-pSer129 (1:800) for 24 hours at 4°C. Secondary Antibody: ATTO 546 Donkey Anti-Mouse (1:700) and ATTO 488 Donkey Anti-Rabbit (1:700) for 1 hour at RT (composite green). Counterstain: Hoechst (blue) nuclear stain at 1:3000 for 1 hour at RT. Localization: Lewy body incluscions. Magnification: 20x.
