Tau Post-Translational Modifications in Alzheimer’s Disease: Diagnostic and Therapeutic Opportunities
The tubulin-associated unit, tau protein, plays a key role in the development of Alzheimer’s disease (AD) and other tauopathies. Normally, the protein is located mainly in axons and is responsible for the stability of the axonal microtubules1. However, under pathological conditions, tau is abnormally hyperphosphorylated and dissociated from microtubules. Abnormal tau aggregates to form neurofibrillary tangles which are insoluble aggregates in the brain and one of the main hallmarks of Alzheimer’s disease2.

Microtubule disintegration caused by the malfunctioning phosphoprotein, tau, which normally stabilizes the microtubules (National Institute on Aging).
Tau post-translational modifications
Post-translational modifications (PTMs) are chemical modifications following protein biosynthesis and play an important role in cellular functions3. While these protein modifications are critical to biological homeostasis some of them can lead to disease progression4. There are several ways tau can be post-translationally modified; the most important include phosphorylation, acetylation, ubiquitination, sumoylation, O-GlcNAcylation, and truncation5.
Tau Phosphorylation
Phosphorylation of tau is the primary mechanism that regulates tau binding to microtubules6. Since hyperphosphorylated tau is the major component of neurofibrillary tangles in Alzheimer’s disease, tau phosphorylation is the most studied post-translational modification and a major pharmacological target in AD therapy2. Hyperphosphorylation occurs due to an imbalance in the catalytic activity of kinases and phosphatases and promotes tau self- aggregation into toxic oligomers and neurofibrillary tangles (NFTs)7.
Phosphorylation is considered a reversible modification which occurs mainly at serine residues of the protein (95%) and in threonine (4%) and tyrosine (1%) residues8. There are at least 85 phosphorylation sites (80 serine/threonine and 5 tyrosine residues). The pSer202/pThr205 residue which is recognized by the AT8 antibody has been studied extensively. A recent study showed that combined phosphorylation at the Ser202/Thr205/Ser208 sites with the absence of phosphorylation at the Ser262 site produces tau that forms fibers and is considered a better epitope for the AT8 antibody9.
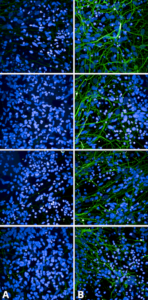
Immunocytochemistry/Immunofluorescence analysis using Rabbit Anti-Tau Monoclonal Antibody, Clone AH36 (SMC-601). SMC-601 recognizes the same epitope as the AT8 phospho tau antibody.
Tau Acetylation
Acetylation, the addition of an acetyl group on lysine residues, is a post-translational modification that regulates normal tau function but also plays a role in tau aggregation in AD10. Several studies have shown that acetylation at specific lysine residues inhibits tau microtubule assembly and enhances tau aggregation10,11. This protein modification is also reversible and catalyzed by histone acetyl transferases and histone deacetylases. An imbalance between these two enzymes can lead to a disruption in homeostasis12. Tau acetylation at Lys174 (K174) is identified as an early pathological change to tau in AD brains. It has been shown to play a crucial role in tau homeostasis and cognitive deficits in a mouse model of tauopathy13.
Tau Ubiquitination
Misfolded or unfolded proteins can be refolded by molecular chaperones (Hsc70/Hsp70 and Hsp40) or degraded by the ubiquitin-proteasome system (UPS). Tau has been shown to undergo ubiquitination by UPS under physiological conditions. UPS dysfunction has been associated with tau aggregation and neurodegeneration in AD. Ubiquitin has been detected in neurofibrillary tangles and senile plaques from AD patients14. The ubiquitin ligase, C-terminus of Hsc70-interacting protein (CHIP), can fold proteins and prevent aggregation. CHIP with HSP70 are able to regulate tau ubiquitination and degradation in a cell culture system. Increased levels of CHIP in tau transgenic mice (P301L) were shown to protect against NFT formation in the early stages of AD in a mouse tauopathy model. This finding shows that ubiquitination might be a clearance mechanism for aggregated tau and a possible therapeutic strategy.15
Tau SUMOylation
SUMOylation is a post-translational modification analogous to ubiquitination but instead of ubiquitin, it involves small ubiquitin-like modifiers (SUMOs) attached to lysine residues of the target protein. In contrast to ubiquitination, SUMOylation does not target proteins for degradation, but regulates protein stability, nuclear-cytosolic transport and transcription. A previous study showed that tau SUMOylation (K340) increases its phosphorylation, enhancing tau aggregation. The data also showed that SUMOylation inhibits ubiquitination of the phosphorylated tau, which may cause tau aggregation16. Inhibition of SUMO modification and promotion of tau ubiquitination could be a promising therapeutic approach.
Tau O-GlcNAcylation
O-GlcNAcylation is the post-translational modification of proteins at the hydroxyl group of serine or threonine residues by O-linked β -N-acetylglucosamine (OGlcNAc). Previous studies showed that in the human brain, tau undergoes O-GlcNAcylation which regulates its phosphorylation. Reduced brain glucose metabolism in AD patients leads to decreased OGlcNAcylation which results to increased tau hyperphosphorylation and aggregation17. Upregulating brain O-GlcNAcylation could potentially be a promising therapeutic strategy for AD and other neurodegenerative diseases18.
Tau Truncation
Truncation of tau has been shown to play a critical role in both tau aggregation and neurodegeneration19. Tau truncation at its N-terminal domain is an important pathogenic mechanism that occurs at the early stages of AD development20. Several specific truncations of tau have been identified in AD brains and are contained in paired helical filaments, such as the dGAE fragment21. Another study demonstrated that caspase-cleavage of tau near the carboxy-terminus is an early event in tau tangle pathology development and the accumulation of these truncated forms in the AD brain could make this PTM an important biomarker in AD diagnosis22.
Tau post-translational modifications in AD diagnosis
Despite the efforts for developing effective therapeutics against Alzheimer’s, little progress has been achieved due to the disease complexity. Researchers are currently focusing on identifying new therapeutic targets and post-translational modifications have drawn great attention. Many pathological proteins in different diseases have dysfunctional PTMs and targeting them could reveal a novel therapeutic strategy. Furthermore, early diagnosis of a disease could be the key to effective treatment.
Since Alzheimer’s disease is characterized by a slowly progressive dementia and brain atrophy, there is a great need for biomarkers that detect the disease early. Currently, there are two different technologies approved for the diagnosis of AD, amyloid-beta positron emission tomography (Aβ-PET), and Aβ and tau measurements in cerebrospinal fluid (CSF). Increased levels of tau in CSF are associated with AD and other dementias but higher levels of phosphorylated tau at T181 (p- tau181) are more specific biomarkers for Alzheimer’s disease23.
An NIH-funded study that was recently published in Nature Medicine presented the development of a blood test that could detect Alzheimer’s disease in people with signs of dementia24. A blood-based test could be less invasive and less expensive than current brain imaging (PET) and CSF tests for AD diagnostics. This blood test detects the abnormal accumulation of p- tau181 which can identify and distinguish AD from other neurological diseases and could be a promising diagnostic biomarker for AD25.
In addition to tau phosphorylation, a wide variety of different PTMs occur on tau protein that are associated with tau aggregation and neurodegeneration. These protein modifications could be exploited to develop novel therapeutic agents and biomarkers. Further studying of these PTMs is the key in understanding the molecular mechanisms associated with AD and also provides the opportunity for early diagnosis and drug development.
StressMarq’s Tau Pre-formed Fibrils (PFFs) (catalog# SPR-471) and monomers (catalog# SPR-473) are post-translationally modified due to the baculovirus/SF9 insect cells expression system.
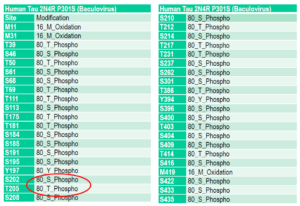
Mass spectrometry analysis of Tau441 (2N4R) P301S mutant PFFs (catalog# SPR-471) expressed in baculovirus. Mass spectrometry analysis shows phosphorylation at sites including threonine 181, serine 202, and threonine 205.
REFERENCES
- A protein factor essential for microtubule assembly, Weingarten MD et al. (1975) Proc Natl Acad Sci U S A. 72(5):1858-62.
- Hyperphosphorylation of microtubule-associated protein tau: a promising therapeutic target for Alzheimer disease. Gong, C., & Iqbal, K. (2008). Cur Med Chem, 15(23), 2321-2328.
- Post-translational chemical modifications of proteins. Kia-Ki, H., & Martinage, A. (1992). International Journal of Biochemistry, 24(1), 19–28.
- Small changes huge impact: the role of protein post-translational modifications in cellular homeostasis and disease. Karve TM, Cheema AK. (2011) J Amino Acids. 2011:207691.
- Degradation or aggregation: the ramifications of post-translational modifications on tau. Park S. et al. (2018) BMB Reports. 51(6):265-273.
- Tau protein modifications and interactions: their role in function and dysfunction. Mietelska-Porowska A. et al. (2014) Int J Mol Sci. 15(3):4671‐4713.
- The importance of tau phosphorylation for neurodegenerative diseases Noble et al. (2013) Front Neurol. 4:83.
- Protein phosphorylation is of fundamental importance in biological Regulation, Nestler EJ et al. (1999) Basic Neurochemistry: Molecular, Cellular and Medical Aspects 6th Edition.
- Epitope mapping and structural basis for the recognition of phosphorylated tau by the anti-tau antibody AT8. Malia T.J. et al. (2016) Proteins. 84:427–434.
- The acetylation of tau inhibits its function and promotes pathological tau aggregation. Cohen T.J. et al. (2011) Nat Commun. 2:252.
- Lysine-Directed Post-translational Modifications of Tau Protein in Alzheimer’s Disease and Related Tauopathies. Kontaxi C. et al. (2017) Front Mol Biosci. 4:56.
- Mass Spectrometry Analysis of Lysine Posttranslational Modifications of Tau Protein from Alzheimer’s Disease Brain. Thomas, S. N. et al. (2017) Methods Mol Biol. 1523:161–177.
- Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Min S.W. et al. (2015) Med.21: 1154-1162.
- The ubiquitin proteasomal system: a potential target for the management of Alzheimer’s disease. Gadhave K. et al. (2016) J Cell Mol Med. 20(7):1392‐1407.
- In vivo evidence of CHIP up-regulation attenuating tau aggregation. Sahara N. et al. (2005) Neurochem.94: 1254-1263.
- SUMOylation at K340 inhibits tau degradation through deregulating its phosphorylation and ubiquitination. Luo H.B. et al. (2014) Proc. Natl. Acad. Sci. USA. 111: 16586-16591.
- O-GlcNAcylation: A regulator of tau pathology and neurodegeneration. Gong C.X. et al. (2016) Alzheimers Dement. 12(10):1078‐1089.
- O-GlcNAcylation as a Therapeutic Target for Alzheimer’s Disease. Park J. et al. (2020) Neuromol Med 22,171–193.
- Tau truncation is a productive posttranslational modification of neurofibrillary degeneration in Alzheimer’s disease. Kovacech B. et al. (2010) Curr Alzheimer Res. 7(8):708‐716.
- N-terminal tau truncation in the pathogenesis of Alzheimer’s disease (AD): Developing a novel diagnostic and therapeutic approach. Amadoro G. et al. (2019) BBA – Molecular Basis of Disease.
- Proteolytic processing of tau. Wang Y. et al. (2010) Soc. Trans.38: 955–961.
- Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. Rissman R.A. et al. (2004) Journal of Clinical Investigation 114(1): 121–130.
- Post-Translational Modifications in Alzheimer’s Disease and the Potential for New Biomarkers. Russell C.L. et al. (2014) Journal of Alzheimer’s Disease 41: 345–364.
- Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Thijssen, E.H. et al. (2020) Nat Med 26, 387–397.
- Another step forward in blood-based diagnostics for Alzheimer’s disease. Bateman R.J. et al. (2020) Nat. Medicine 26: 314-316.


Leave a Reply