VPS35 in Parkinson’s Disease
Parkinson’s disease is a complex neurodegenerative condition, characterized by movement disorders such as a resting tremor and postural instability, and non-motor syndromes like dementia and disrupted sleep. It is the second most common neurodegenerative disease after Alzheimer’s, yet in 90% of cases the cause is unknown1. The remaining 10% of cases are inherited and have been linked to mutations in several genes2. Of these, the gene encoding vacuolar protein sorting-associated protein 35 (VPS35, also referred to as vesicle protein sorting 35) has been definitively correlated to familial Parkinson’s disease following the identification of a point mutation (D620N) in the DNA of multiple closely-related individuals affected with a late onset form of the condition3,4. Since this discovery, research efforts have focused on understanding the physiological role of VPS35 and the involvement of the D620N mutation in Parkinson’s disease.
What is the role of VPS35 in Parkinson’s disease?
VPS35 was first identified in yeast over 20 years ago, when it was shown to be a component of the retromer complex5. Following its association with VPS26 and VPS29 to form a trimer, a second sub-complex comprising VPS5/VPS17 is recruited. The resulting retromer complex plays an essential role in transporting proteins from endosomes to the Golgi apparatus, including the trafficking of several receptors. Because receptor endocytosis is an important mechanism to attenuate signaling, retromer complex dysfunction is proposed to drive the pathogenesis of various neurodegenerative disorders.
Many studies have been performed to investigate the role of mutated VPS35 in retromer complex dysfunction and Parkinson’s disease. A germline D620N VPS35 knock-in mouse model has demonstrated the possibility of a link between mutant VPS35 and tau for inducing neurodegeneration, whereas the D620N mutation has been shown to provide a gain of function of LRRK2 activity, potentially causing Parkinson’s disease through hyperactivation of the LRRK2 kinase6,7. Researchers have also demonstrated over-expression of D620N VPS35 in rat primary cortical neurons to induce neuronal cell death and impair neurite outgrowth8.
To date, three potential cellular mechanisms have been suggested to underpin mutant VPS35-induced neurodegeneration. These are impaired binding of the retromer complex to the WASH complex, resulting in compromised autophagy; disrupted AMPA receptor trafficking, leading to the disruption of essential neuronal activities; and altered mitochondrial dynamics and function, causing mitochondrial fragmentation and neuronal loss9. Research efforts are ongoing to better understand Parkinson’s disease pathogenesis and ultimately develop an effective treatment.
Supporting the study of VPS35 in Parkinson’s disease
We provide an extensive selection of reagents to support the study of VPS35 and its role in Parkinson’s disease. These include our anti-VPS35 antibody (SMC-602), which has been validated in VPS35 knockout A549 cells, and our VPS35 ELISA kit (SKT-141), for colorimetric detection of VPS35 in cell lysate and tissue samples.
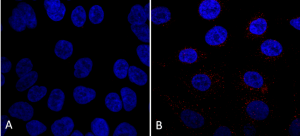
ICC/IF analysis using Mouse Anti-VPS35 Monoclonal Antibody, Clone 7E4 (SMC-602). Tissue: A549 cells. Species: Human. Primary Antibody: Mouse Anti-VPS35 Monoclonal Antibody (SMC-602) at 1:5 (tissue culture supernatant). Secondary Antibody: Donkey anti-mouse: Alexa Fluor 594 at 1:4000 in 0.2% BSA PBS. Counterstain: DAPI. Localization: Vesicles. A) VPS35 KO A549 cells B) WT A549 cells. Courtesy of Dario Alessi Lab, University of Dundee.
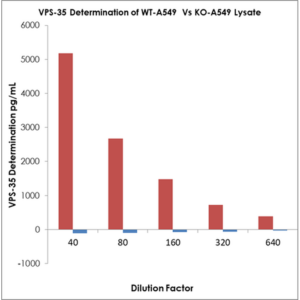
VPS35 determination of WT-A549 vs KN-549 lysates using the VPS35 kit (SKT-141). MOLECULAR SIGNATURE®, assay type: competitive ELISA, detection method: colorimetric.
REFERENCES
- Contributions of VPS35 Mutations to Parkinson’s Disease, Rahman AA and Morrison BE, Neuroscience. 2019 Mar 1;401:1-10
- Genetics in Parkinson disease: Mendelian versus non-Mendelian inheritance, Hernandez DG et al, J Neurochem. 2016 Oct;139 Suppl 1:59-74
- VPS35 mutations in Parkinson disease, Vilariño-Güell C et al, Am J Hum Genet. 2011 Jul 15;89(1):162-7
- A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease, Zimprich A et al, Am J Hum Genet. 2011 Jul 15;89(1):168-75
- A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast, Seaman MN et al, J Cell Biol. 1998 Aug 10;142(3):665-81
- Parkinson’s disease-linked D620N VPS35 knockin mice manifest tau neuropathology and dopaminergic neurodegeneration, Chen X et al, Proc Natl Acad Sci U S A. 2019 Mar 19;116(12):5765-5774
- The Parkinson’s disease VPS35[D620N] mutation enhances LRRK2-mediated Rab protein phosphorylation in mouse and human, Mir R et al, Biochem J. 2018 Jun 6;475(11):1861-1883
- Parkinson’s disease-linked mutations in VPS35 induce dopaminergic neurodegeneration, Tsika E et al, Hum Mol Genet. 2014 Sep 1;23(17):4621-38
- VPS35, the Retromer Complex and Parkinson’s Disease, Williams ET et al, J Parkinsons Dis. 2017;7(2):219-233

Leave a Reply